When you pick up a prescription for insulin or a biologic like Humira, you might not realize your pharmacist could swap it for a cheaper version without telling you. That’s not a mistake-it’s interchangeability. In the U.S., some biosimilars are legally allowed to be swapped automatically at the pharmacy, just like generic pills. But here’s the catch: not all biosimilars can do this. Only a handful have earned the FDA’s special interchangeability stamp. And even then, whether you get swapped depends on where you live.
What Makes a Biosimilar Interchangeable?
Biosimilars aren’t like regular generics. You can’t just swap a brand-name pill for a generic one and call it done. Biologics-drugs made from living cells-are incredibly complex. Even tiny changes in how they’re made can affect how they work in your body. That’s why the FDA requires way more proof for a biosimilar to be called interchangeable. To get that label, a company must run switching studies. Not just one switch-multiple. Patients get switched back and forth between the original drug and the biosimilar, sometimes four or five times. The goal? Prove that each switch doesn’t cause more side effects, reduce effectiveness, or create new safety risks. The FDA’s 2023 guidance says these studies need to be done in a homogeneous group of patients, with clear measurements of how the drug moves through the body and how it affects targets like inflammation. It’s not about being "better"-it’s about being switchable. All FDA-approved biosimilars are just as safe and effective as the original. But only those that pass the switching test can be swapped automatically. As of November 2023, out of 41 approved biosimilars in the U.S., only 10 have interchangeability status. The first was Semglee, an insulin glargine product approved in July 2021. The first interchangeable monoclonal antibody? Cyltezo, approved in August 2023, a copy of Humira.Why Does This Even Matter?
Biologics are expensive. A single dose of Humira can cost over $2,000. Biosimilars typically cost 15-30% less. That’s thousands of dollars a year in savings. But if pharmacists can’t swap them without calling the doctor, uptake stays low. Interchangeability removes that barrier. It lets the market work. States with automatic substitution laws saw 18.7% higher biosimilar use for insulin, according to a 2023 JAMA Health Forum study. Semglee hit a 17.3% market share within six months-nearly double the pace of non-interchangeable biosimilars. That’s the power of automatic substitution. It’s not just about cost. It’s about access. For patients on long-term biologics for rheumatoid arthritis, psoriasis, or Crohn’s disease, lower prices mean fewer people skip doses or drop treatment entirely. But here’s the problem: interchangeability doesn’t mean you can swap between biosimilars. If you’re on one biosimilar and your pharmacy tries to switch you to another, that’s not allowed under FDA rules. Only substitution back to the original reference product is permitted. That’s confusing for pharmacists and patients alike.State Laws Make It a Patchwork
Federal rules say interchangeability is possible. But state laws decide if it happens. Forty states let pharmacists swap interchangeable biosimilars without asking the doctor. Arizona, for example, lets pharmacists substitute as long as they notify the patient, record the product, and send a note to the prescriber within five days. But six states and D.C. only allow substitution if it saves the patient money. Four states-Alabama, Indiana, South Carolina, and Washington-require the doctor’s approval every time. And Puerto Rico does too. That’s a nightmare for national pharmacy chains. A pharmacist in California might need to check if the swap lowers the patient’s out-of-pocket cost. In Arizona, they don’t. But their pharmacy software doesn’t always tell them which state’s rules apply. A 2022 survey by the National Community Pharmacists Association found that 67% of independent pharmacists felt confused about state rules. One Reddit user wrote: "I spent 45 minutes last week trying to figure out if I could substitute Hadlima in Oregon. My system didn’t flag it. I called the prescriber anyway, just to be safe."
What Patients Are Saying
Patient experiences are mixed. On the Psoriasis Foundation forum, one person said: "Switched from Humira to Hyrimoz. Saved $800 a month. No difference in my skin." Another wrote: "My pharmacy swapped my Humira for Hadlima without telling me. I broke out in a rash. Turns out I was allergic to an excipient-something not in the original." A 2022 National Psoriasis Foundation survey found 63% of patients were satisfied with their biosimilar. But 28% were upset they weren’t told about the switch. That’s a big deal. Patients want to be involved in their care. Even if the drug is safe, the lack of communication breeds distrust. Pharmacists are trying to do better. The American Pharmacists Association has trained over 12,000 pharmacists through a 2.5-hour Biosimilars Certificate Program. Still, the average pharmacist spends nearly nine hours a year just keeping up with changing rules.Interchangeable vs. Generic: The Key Difference
People often think biosimilars are just "biologic generics." They’re not. Generics are chemically identical to their brand-name counterparts. A 500mg tablet of generic amoxicillin is the same molecule as the brand version. Biosimilars are highly similar-but not identical. They’re made in living cells, so slight variations are unavoidable. That’s why they need extra proof for interchangeability. The Hatch-Waxman Act lets pharmacists swap generics automatically. The BPCIA, passed in 2010, created the separate path for biosimilars. It’s a more cautious approach, built on the complexity of biologics. The FDA doesn’t treat interchangeability as a "higher tier" of biosimilar. It’s just a different permission slip for substitution.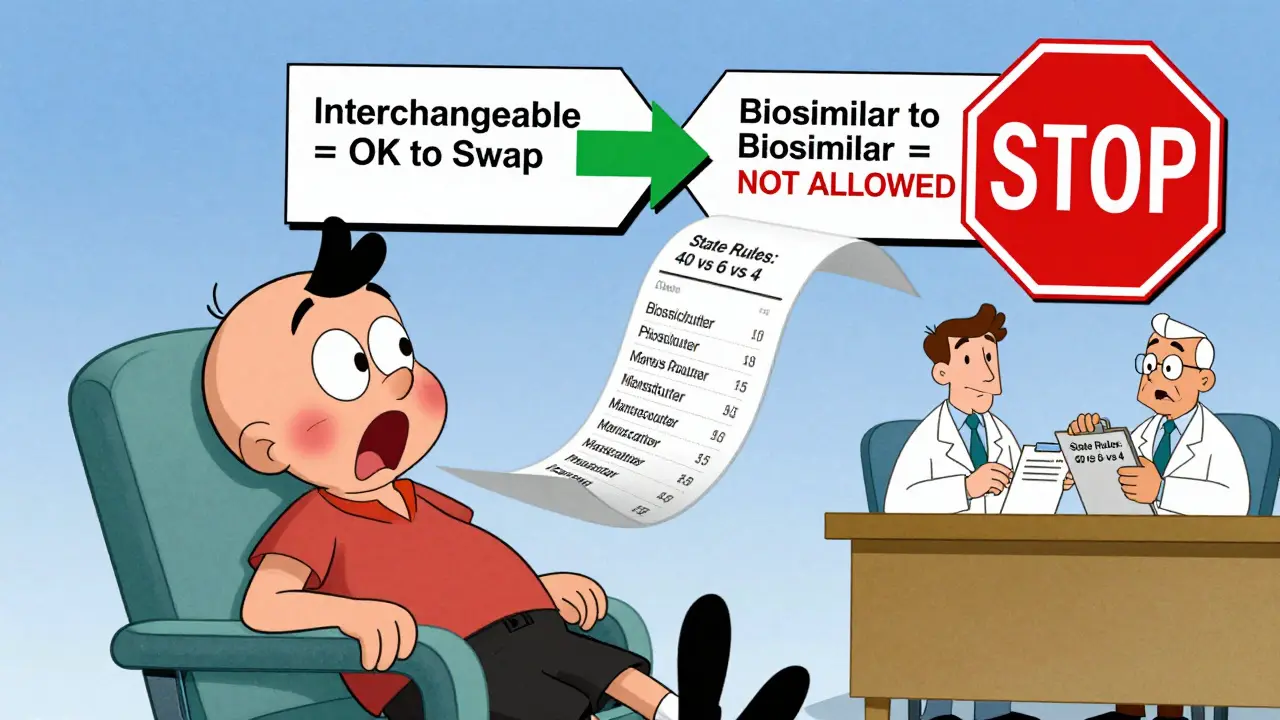
The Future: Will All Biosimilars Become Interchangeable?
There’s a bill in Congress called the Biosimilar Red Tape Elimination Act. It would scrap the switching study requirement and make every FDA-approved biosimilar automatically interchangeable. Proponents say it’s the fastest way to slash costs. The Biosimilars Council supports it. But PhRMA, the big pharma lobby, warns it could hurt safety. They point to a 2021 JAMA Dermatology study showing psoriasis patients switched to biosimilars had a 20.3% higher chance of stopping treatment. The FDA hasn’t taken a side yet. But in 2023, they released draft guidance suggesting they might streamline the switching study process-not eliminate it. That’s a middle path: keep the safety checks, but make them less burdensome. By 2026, biosimilars could capture nearly half the $168 billion biologics market, according to Evaluate Pharma. But that future depends on whether patients, pharmacists, and doctors can navigate the current chaos. Right now, the system works-but only if everyone understands the rules.What You Should Do
If you’re on a biologic:- Ask your doctor if your drug has an interchangeable biosimilar.
- Check with your pharmacist: Will they substitute it automatically? Do they need your permission?
- Know your state’s rules. If you live in Alabama or Washington, your doctor’s signature is required. In Arizona, they can swap it-but must tell you.
- Always read the label. If the manufacturer name changed, ask why.
- Report any side effects after a switch. Even if you think it’s minor.
- Use "dispense as written" on prescriptions if you want to block substitution.
- Know the DAW codes your pharmacy system uses.
- Don’t assume your patient knows the difference between a biosimilar and a generic.
Can my pharmacist switch my biologic without telling me?
It depends on your state and whether your drug has an interchangeable biosimilar. In 40 states, pharmacists can swap interchangeable biosimilars without telling you. But they’re required to notify you in states like Arizona. In four states-including Alabama and Washington-they need your doctor’s approval first. Always check your prescription label for the manufacturer name. If it changed, you were likely switched.
Are interchangeable biosimilars safer than regular biosimilars?
No. All FDA-approved biosimilars-interchangeable or not-are held to the same high standards for safety and effectiveness. The only difference is that interchangeable ones have passed extra studies showing you can switch back and forth between them and the original drug without added risk. It’s not a higher level of quality-it’s a different permission for substitution.
Can I be switched from one biosimilar to another?
No. FDA rules only allow substitution between a reference product and its interchangeable biosimilar. If you’re on one biosimilar and your pharmacy tries to switch you to another biosimilar of the same drug, that’s not permitted under current guidelines. That’s a common point of confusion. Always confirm the exact product name on your prescription and pharmacy label.
Why are there so many different state laws?
The federal government set the rules for interchangeability, but states control how prescriptions are dispensed. That means each state can add its own rules-like requiring cost savings, patient notification, or prescriber approval. This creates a patchwork that’s hard for pharmacies to manage. It’s why many pharmacists spend nearly 9 hours a year just learning the rules.
What’s the difference between a biosimilar and a generic?
Generics are exact chemical copies of brand-name drugs. Biosimilars are highly similar to biologic drugs but not identical, because biologics are made from living cells, not synthesized chemicals. That’s why biosimilars need more testing to prove they’re safe. Only some biosimilars can be swapped automatically-generics can always be swapped.


Betty Bomber
January 27, 2026 AT 19:42So let me get this straight - my pharmacist can swap my Humira for something cheaper without telling me? In Arizona? That’s wild. I’d never know unless I checked the label. My mom’s on biologics and she’d flip if she found out. No warning, no consent. Feels like a bait-and-switch.
Sally Dalton
January 28, 2026 AT 22:11OMG YES I JUST GOT SWITCHED TO SEMGLEE AND DIDN’T EVEN NOTICE UNTIL I SAW THE BOX 😱 I THOUGHT I WAS GETTING THE SAME THING! I’M FINE WITH THE SAVINGS (LIKE $700/MONTH??) BUT WHY NO TELL? I’M NOT A ROBOT. I WANT TO KNOW WHAT’S GOING IN MY BODY. ALSO MY SKIN’S BEEN GOOD SO FAR BUT I’M STILL NERVOUS. 🤞
Neil Thorogood
January 30, 2026 AT 10:28Y’all act like this is some scandal. 🤡 The FDA says it’s safe, the studies say it’s safe, and your wallet says it’s smart. If you can’t handle a biosimilar because you’re scared of a different label, maybe you’re not ready for modern medicine. Also, if your pharmacy software can’t tell you the state rules, maybe you should quit being a pharmacist and go sell insurance. 😎
Allie Lehto
February 1, 2026 AT 09:35This is why America is crumbling. People are being swapped like commodities. No consent. No dignity. Where’s the humanity? We’ve turned healthcare into a Walmart clearance aisle. My grandmother died because they switched her meds without telling her. This isn’t innovation - it’s negligence dressed up as savings. 🙏
Napoleon Huere
February 2, 2026 AT 01:01Think about it - we’ve built a system where a molecule made in a living cell can be swapped like a battery, but we still can’t agree on whether a pharmacist needs to say ‘hi’ before doing it. Interchangeability isn’t just about science. It’s about trust. And right now, trust is the scarcest drug in the system.
Ryan W
February 3, 2026 AT 09:5440 states allow substitution? Pathetic. The feds should mandate nationwide interchangeability. Why are we letting state bureaucrats dictate medical logistics? This patchwork is a regulatory nightmare designed by lobbyists who want to keep prices high. If the FDA says it’s safe, let it fly. Stop the state-level petty tyranny. #FreeTheBiosimilars
Simran Kaur
February 4, 2026 AT 10:35In India, we don’t have this problem - biosimilars are everywhere and everyone knows what they are. We call them ‘copy drugs’ and doctors explain them clearly. No confusion. No hidden switches. Maybe the U.S. needs less bureaucracy and more education. Patients aren’t stupid - they just need to be told the truth. And yes, I saved 80% on my rheumatoid arthritis meds. No rash. Just relief.
Shweta Deshpande
February 4, 2026 AT 16:53Hi everyone! I just wanted to say I’m so glad this topic is getting attention. I’ve been on Humira for 7 years and switched to Cyltezo last year - no issues at all! My insurance paid $20 instead of $1,200. I cried when I saw the receipt. But I also know people who had reactions, and I get it - change is scary. The key is communication. If pharmacists took 30 seconds to say, ‘Hey, your drug changed but it’s safe,’ it would make all the difference. Maybe we need a simple handout or QR code on the bottle? Just something to say, ‘You’re not alone in this.’ 💛
Shawn Raja
February 5, 2026 AT 06:27Let’s be real - the whole ‘switching studies’ thing is just Big Pharma’s way of slowing down competition. They want you to think biosimilars are risky. They’re not. They’re just cheaper. The FDA doesn’t care if you switch 5 times or 50 times - they care if you live or die. And guess what? People live. They thrive. The real problem? We’ve turned medicine into a legal chess match instead of a healing practice. Fix the system, not the paperwork.
bella nash
February 6, 2026 AT 16:14It is not the mere presence of interchangeability that constitutes the ethical imperative of pharmaceutical policy but rather the epistemological framework underpinning its implementation. The ontological status of the biosimilar as a proximate but nonidentical entity necessitates a hermeneutic of transparency that transcends mere regulatory compliance. One must interrogate not only the clinical efficacy but also the phenomenological experience of the patient in the context of an opaque substitution paradigm. Without informed consent, even the most statistically benign intervention becomes an existential violation.